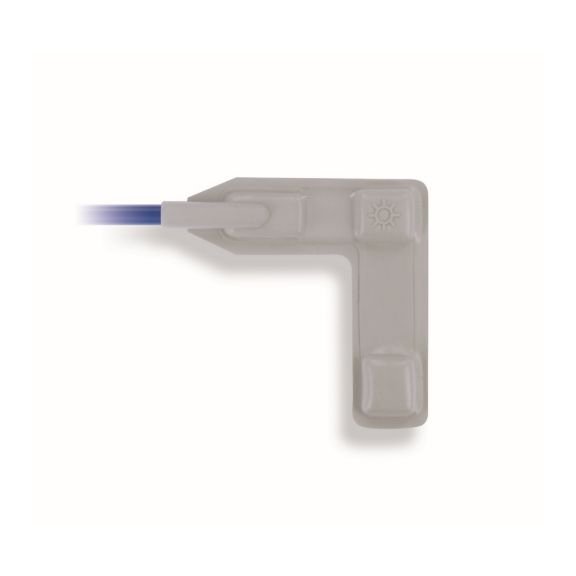
Reusable Pulse Oximetry Sensors
Wrap Sensors
Reusable wrap pulse oximetry sensors for neonates to adults. Flexible application. Easy to clean
EnviteC Cross Reference List | Tech Support
Reusable wrap pulse oximetry sensors that wrap. Easy to clean and offer flexible applications
EnviteC by Honeywell