Disposable Pulse Oximetry Sensors
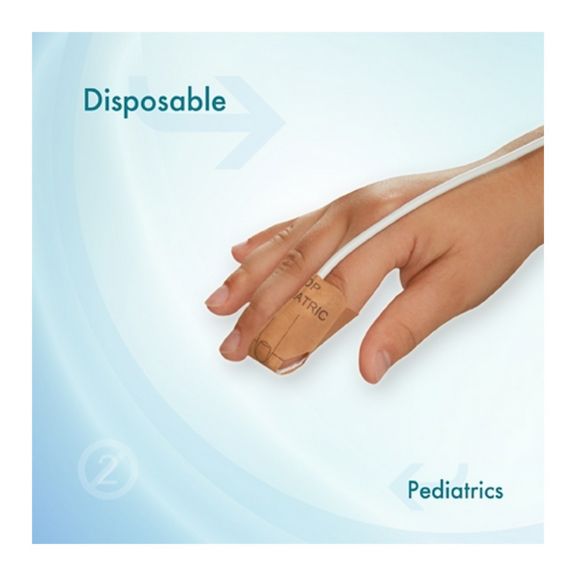
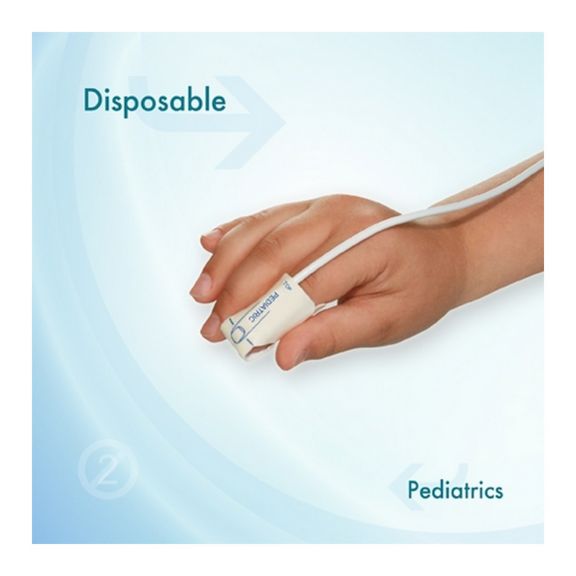
Pediatric Disposable Pulse Oximetry Sensors
Disposable pulse oximetry sensors for children. Comfort in re-positioning (soft medical grade tape). Less adhesive-related skin trauma where necessary – neonates, geriatric patients, patients with burn injuries and adapted for environment
EnviteC Cross Reference List | Tech Support
Disposable SpO2 sensors for children. These sensors do not entail the risk of cross-contamination caused by medical products that are re-used from patient to patient.
The high flexibility, adaptability and bio-compatibility of the applied soft material makes this sensor type ideal for applications where the patient needs extended long-term monitoring.
EnviteC by Honeywell