Oxímetro de pulso y accesorios
Pulsioxímetro y accesorios
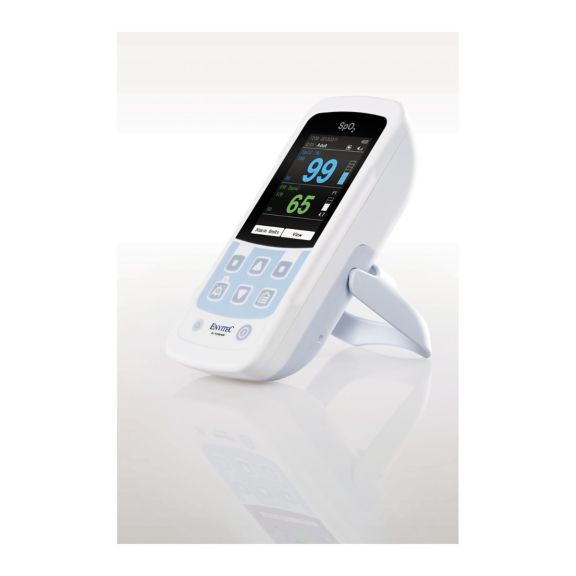
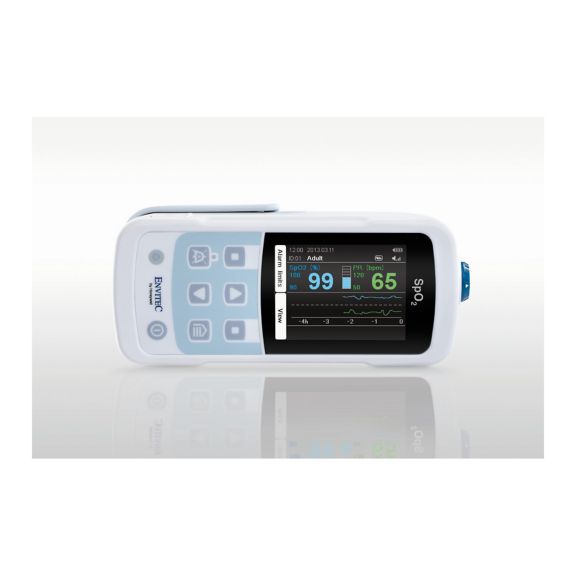
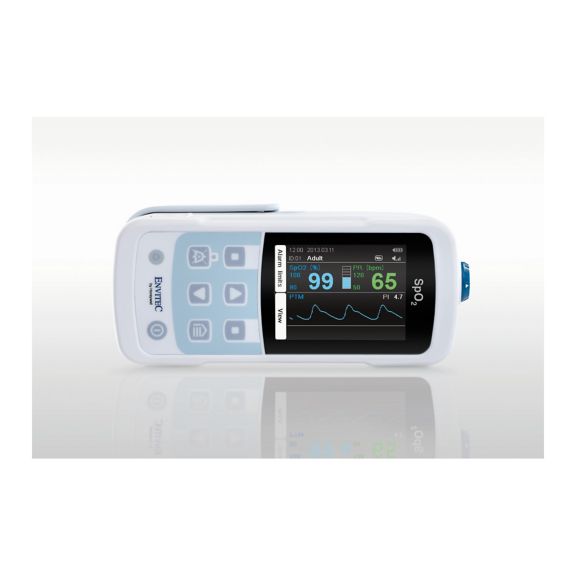
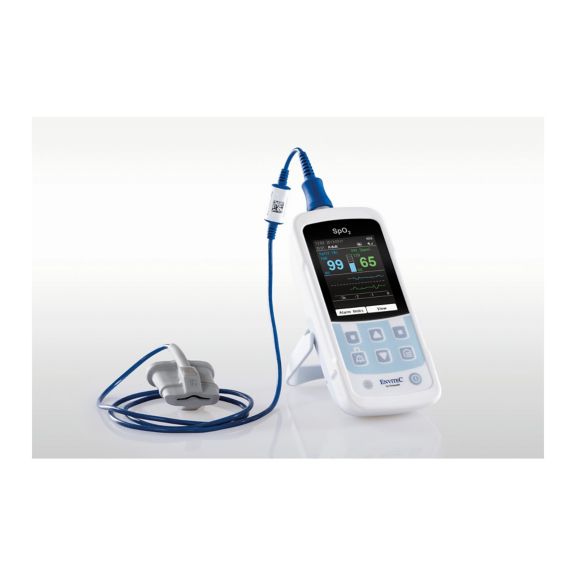
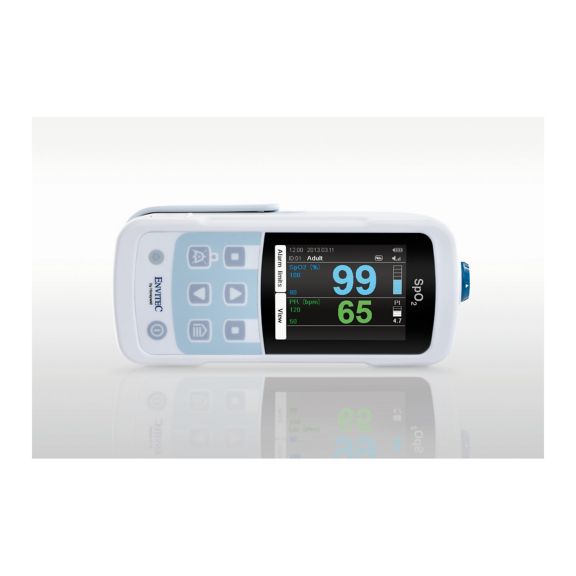
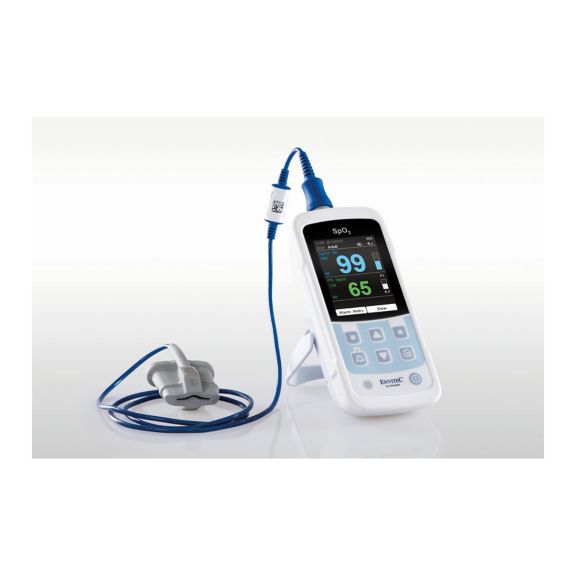
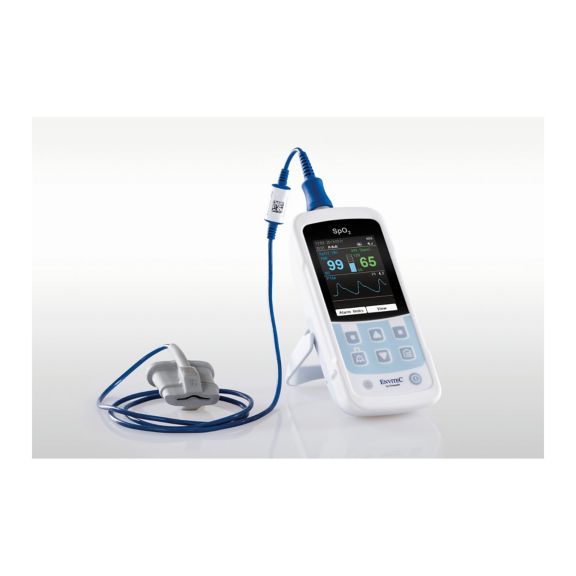
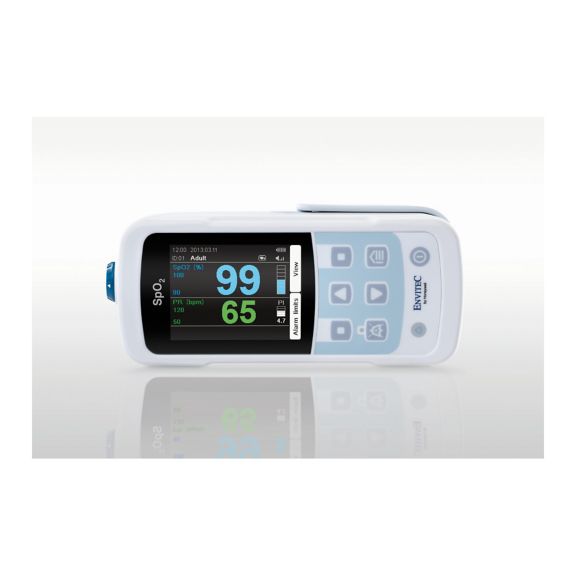
Un dispositivo de pulsioxímetro: muchas opciones. Adecuado para la monitorización continua y puntual de SpO2. Diseñado como dispositivo portátil o estacionario. Mide la SpO2, la frecuencia del pulso y muestra la onda de pulso pletismográfica.
Lista de referencias cruzadas de EnviteC | Soporte técnico
La oximetría de pulso es una tecnología no invasiva, simple y confiable que mide la saturación funcional de oxígeno de la sangre arterial (SpO2) y la frecuencia del pulso.
La disponibilidad de monitores de oxímetro de pulso pequeños, portátiles y de mano ofrece numerosas y diferentes áreas de aplicación en entornos sanitarios. Los sensores de SpO2 son el elemento central en la práctica. Se aplican a las partes del cuerpo correspondientes del paciente.
MySign® S es una solución inteligente para el monitoreo no invasivo de los signos vitales: un monitor fácil de usar que proporciona lecturas excepcionalmente precisas.
EnviteC by Honeywell