Sensores de pulsioximetría reutilizables
SoftTip®
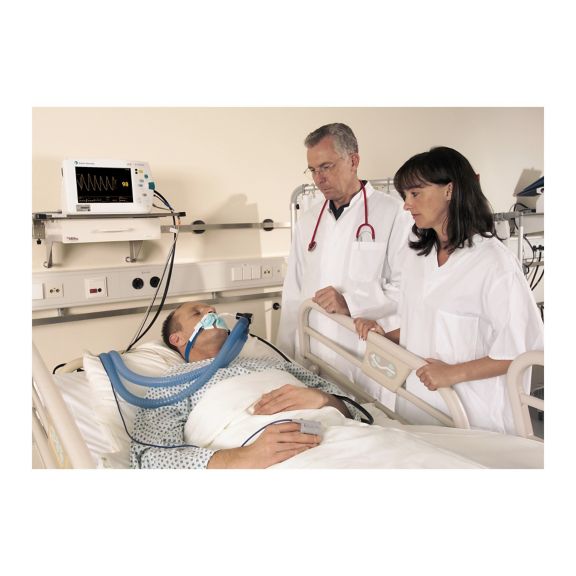
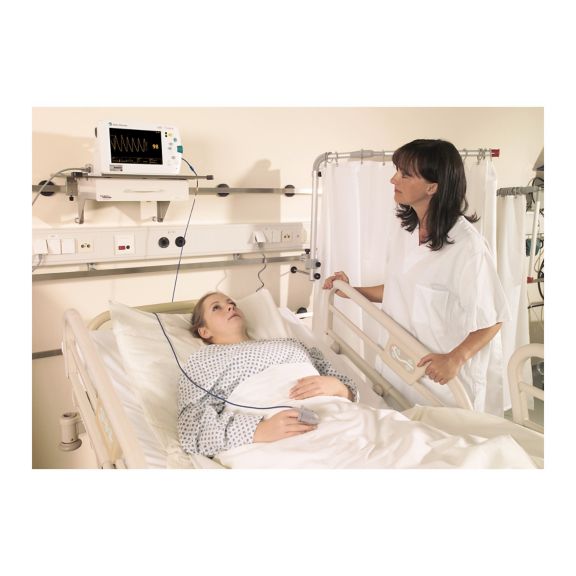
Sensores de pulsioximetría reutilizables SoftTip® con fácil manejo, limpieza óptima y desinfección exhaustiva. Máxima resistencia al estrés mecánico. Gran comodidad de uso gracias a los diversos tamaños para todas las aplicaciones
Lista de referencias cruzadas de EnviteC | Soporte técnico
Un sensor del dedo reutilizable a largo plazo que proporciona una limpieza fácil por inmersión completa en líquido limpiador. Además, soporta el máximo estrés mecánico.
El diseño único del producto garantiza una comodidad del paciente extraordinariamente alta y resultados de medición excepcionalmente precisos. Se garantiza la compatibilidad con los dispositivos de monitorización habituales de la mayoría de fabricantes conocidos.
EnviteC by Honeywell