一次性脉搏血氧测定传感器
适合新生儿用的一次性脉搏血氧测定传感器
适合新生儿用的一次性脉搏血氧测定传感器。可重新定位以改善舒适度(柔软的医用级胶带)。对于新生儿、老年患者、烧伤患者,可减少与粘合剂相关的皮肤创伤,适应不同环境
适合新生儿用的一次性 SpO2 传感器。这些传感器不会因患者之间重复使用医疗产品而引发交叉污染风险。
传感器所用的软材料具有高灵活性、适应性和生物兼容性,非常适合患者需要长期监测的应用。
霍尼韦尔的 EnviteC
一次性脉搏血氧测定传感器
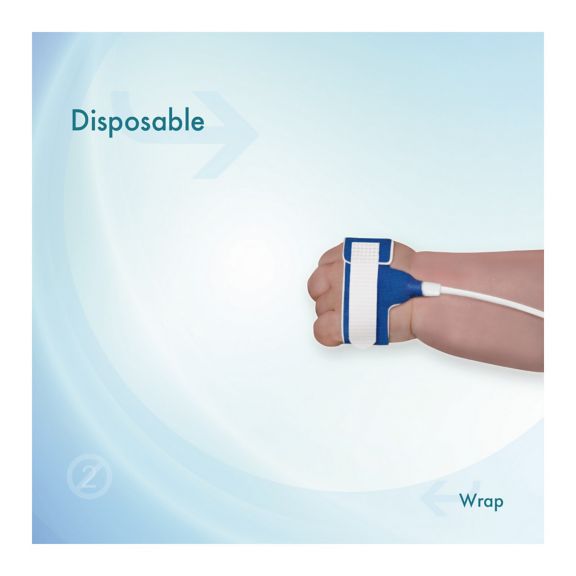

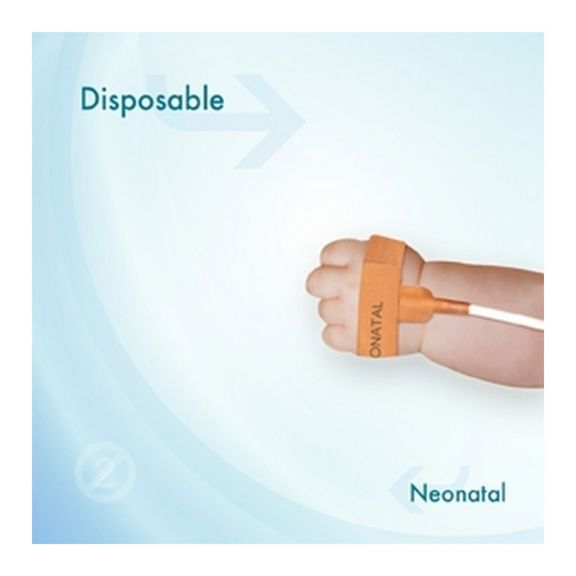
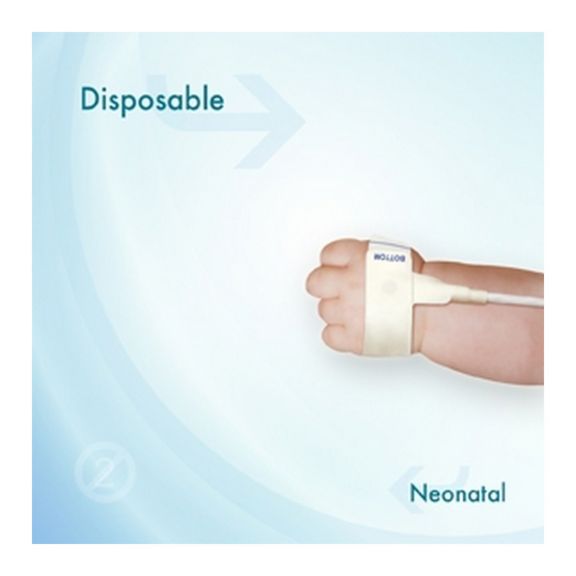
适合新生儿用的一次性 SpO2 传感器。这些传感器不会因患者之间重复使用医疗产品而引发交叉污染风险。
传感器所用的软材料具有高灵活性、适应性和生物兼容性,非常适合患者需要长期监测的应用。
霍尼韦尔的 EnviteC
订阅我们!
立即注册以通过电话、邮件等电子通信方式接收霍尼韦尔产品更新、技术信息、新品发布、活动和新闻、问卷调查、特别优惠等相关主题的独家营销信息。
版权所有 ©2024 Honeywell International Inc
