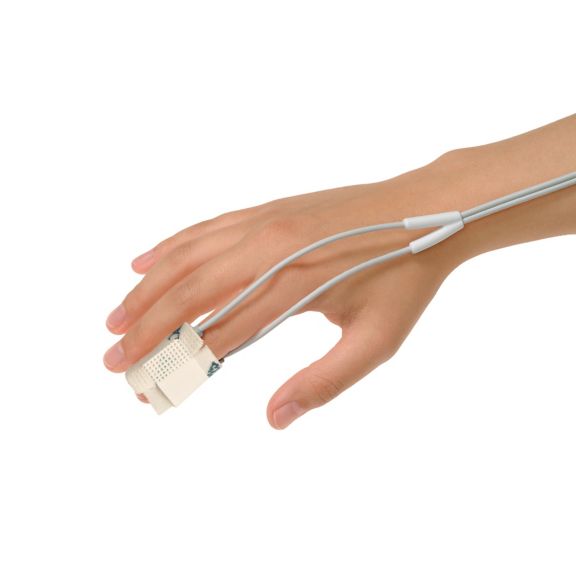
可重复使用的脉搏血氧测定传感器
总览
名称
描述
文件大小
日期
大小
SpO2 Sensors and Monitors Brochure
application/pdf 1.18 MB
9/22/2021
1.18 MB
SpO2 Sensors and Monitors Brochure
application/pdf 1.19 MB
9/22/2021
1.19 MB
名称
描述
文件大小
日期
大小
Form certificate on hygiene status and desinfection of returned product/s
application/pdf 470.7 KB
9/22/2021
470.7 KB
Manufacturers Declaration according to EU MDR.
application/pdf 215.73 KB
6/27/2024
215.73 KB
Confirmation statement about company renaming
application/pdf 227.43 KB
6/26/2024
227.43 KB
Confirmation Letter on validity of EC Certificate (MDD).
application/pdf 405.61 KB
6/27/2024
405.61 KB
Certificate According To ISO 13485 (English)
application/pdf 340.29 KB
6/26/2024
340.29 KB
Form certificate on hygiene status and desinfection of returned product/s
application/pdf 469.94 KB
9/22/2021
469.94 KB
名称
描述
文件大小
日期
大小
EC-Conformity Statement for Reusable Pulse Oximetry Sensors - Y Sensors
application/pdf 346.46 KB
9/23/2021
346.46 KB
Form details on potential safety relevant failures or events
application/pdf 495.94 KB
9/22/2021
495.94 KB
Form details on potential safety relevant failures or events
application/pdf 492.27 KB
9/22/2021
492.27 KB
EC-Conformity Statement for Reusable Pulse Oximetry Sensors - Y Sensors
application/pdf 139.58 KB
9/23/2021
139.58 KB
名称
描述
文件大小
日期
大小
Form certificate on hygiene status and desinfection of returned product/s
application/pdf 470.7 KB
9/22/2021
470.7 KB
Manufacturers Declaration according to EU MDR.
application/pdf 215.73 KB
6/27/2024
215.73 KB
Confirmation statement about company renaming
application/pdf 227.43 KB
6/26/2024
227.43 KB
Confirmation Letter on validity of EC Certificate (MDD).
application/pdf 405.61 KB
6/27/2024
405.61 KB
Certificate According To ISO 13485 (English)
application/pdf 340.29 KB
6/26/2024
340.29 KB
SpO2 Sensors and Monitors Brochure
application/pdf 1.18 MB
9/22/2021
1.18 MB
EC-Conformity Statement for Reusable Pulse Oximetry Sensors - Y Sensors
application/pdf 346.46 KB
9/23/2021
346.46 KB
SpO2 Sensors and Monitors Brochure
application/pdf 1.19 MB
9/22/2021
1.19 MB
Form details on potential safety relevant failures or events
application/pdf 495.94 KB
9/22/2021
495.94 KB
Form details on potential safety relevant failures or events
application/pdf 492.27 KB
9/22/2021
492.27 KB
Form certificate on hygiene status and desinfection of returned product/s
application/pdf 469.94 KB
9/22/2021
469.94 KB
EC-Conformity Statement for Reusable Pulse Oximetry Sensors - Y Sensors
application/pdf 139.58 KB
9/23/2021
139.58 KB
名称
描述
文件大小
日期
大小
Form certificate on hygiene status and desinfection of returned product/s
470.7 KB
9/22/2021
Manufacturers Declaration according to EU MDR.
215.73 KB
6/27/2024
Confirmation statement about company renaming
227.43 KB
6/26/2024
Confirmation Letter on validity of EC Certificate (MDD).
405.61 KB
6/27/2024
Certificate According To ISO 13485 (English)
340.29 KB
6/26/2024
Form certificate on hygiene status and desinfection of returned product/s
469.94 KB
9/22/2021
EC-Conformity Statement for Reusable Pulse Oximetry Sensors - Y Sensors
346.46 KB
9/23/2021
Form details on potential safety relevant failures or events
495.94 KB
9/22/2021
Form details on potential safety relevant failures or events
492.27 KB
9/22/2021
EC-Conformity Statement for Reusable Pulse Oximetry Sensors - Y Sensors
139.58 KB
9/23/2021
订阅我们!
立即注册以通过电话、邮件等电子通信方式接收霍尼韦尔产品更新、技术信息、新品发布、活动和新闻、问卷调查、特别优惠等相关主题的独家营销信息。
版权所有 ©2024 Honeywell International Inc