Einweg-Pulsoximetriesensoren
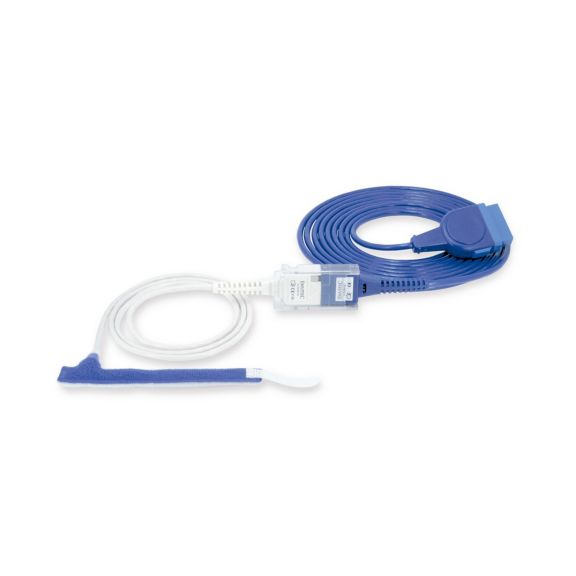
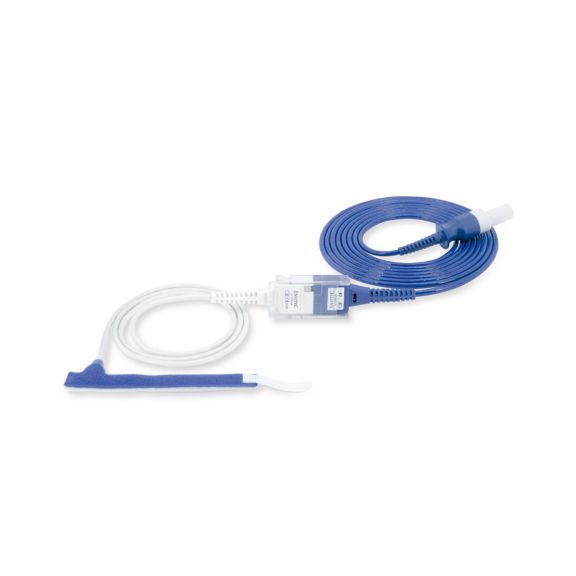
Wrap-Sensor
Wiederverwendbare Wrap-Pulsoximetriesensoren für Neugeborene bis Erwachsene. Flexible Anwendung. Leicht zu reinigen
EnviteC-Querverweisliste | Technische Unterstützung
Wiederverwendbare Wrap-Pulsoximetriesensoren. Leicht zu reinigende und flexible Anwendungen.
EnviteC by Honeywell