Pulsoximeter und Zubehör
Pulsoximeter und Zubehör
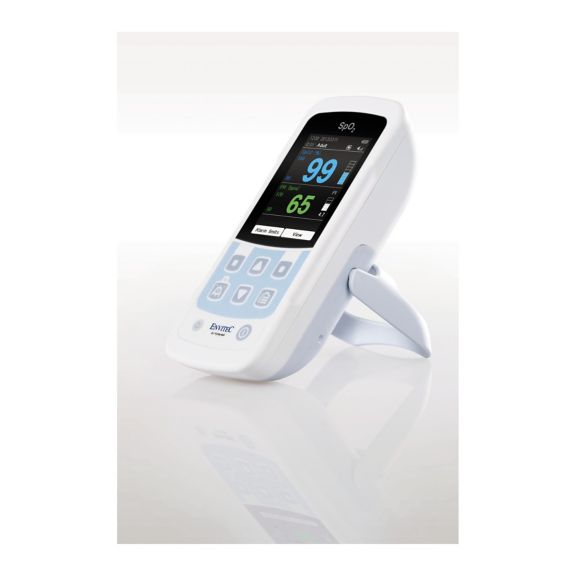
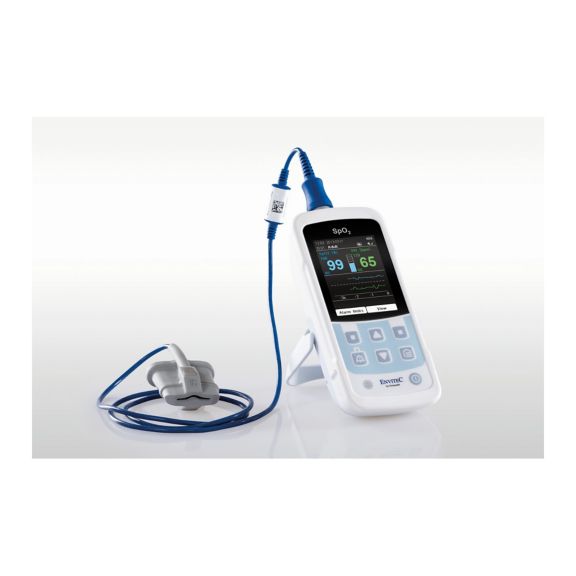
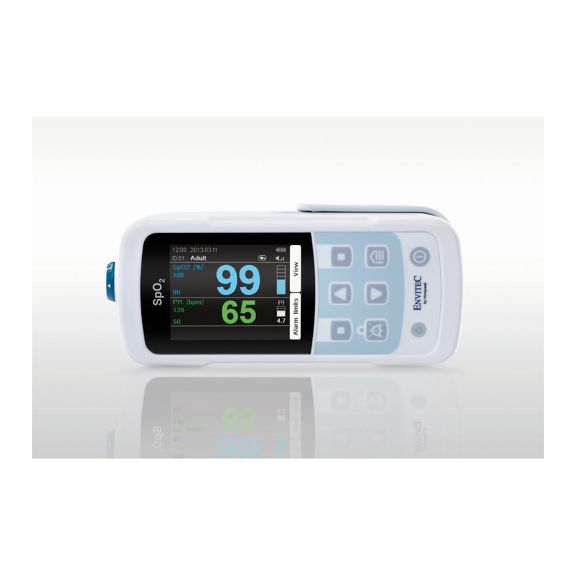
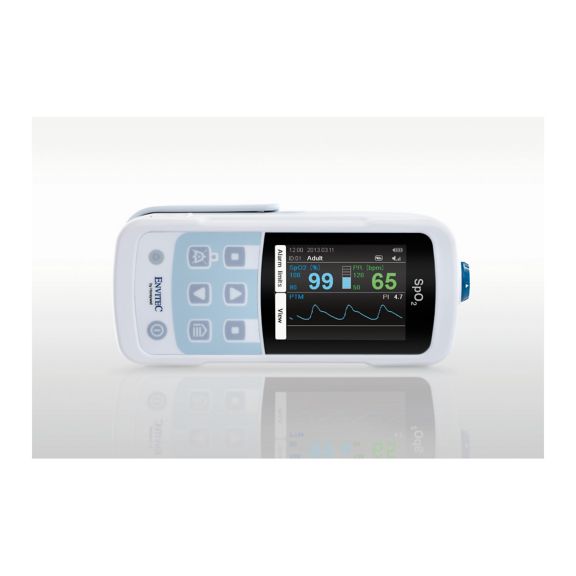
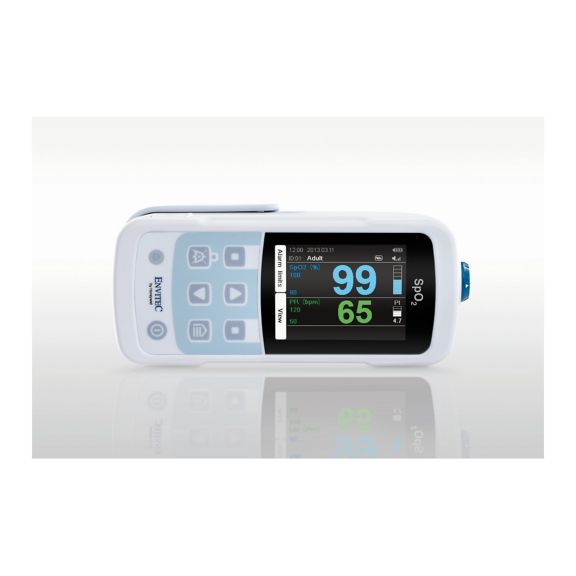
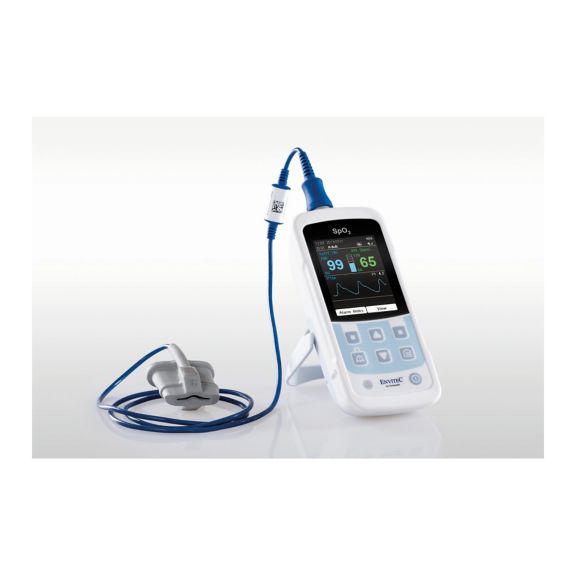
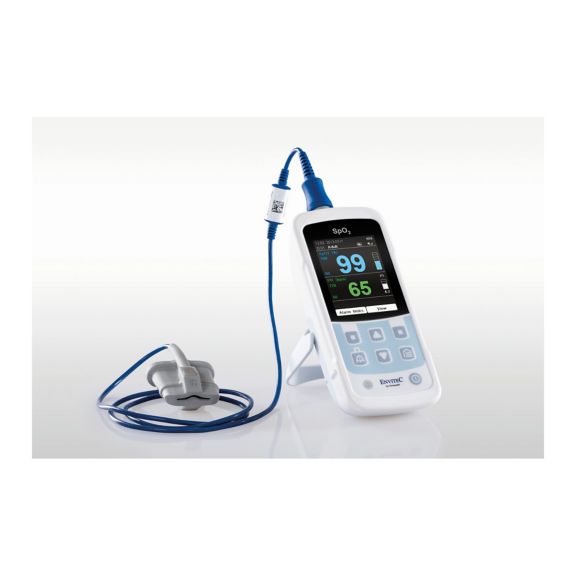
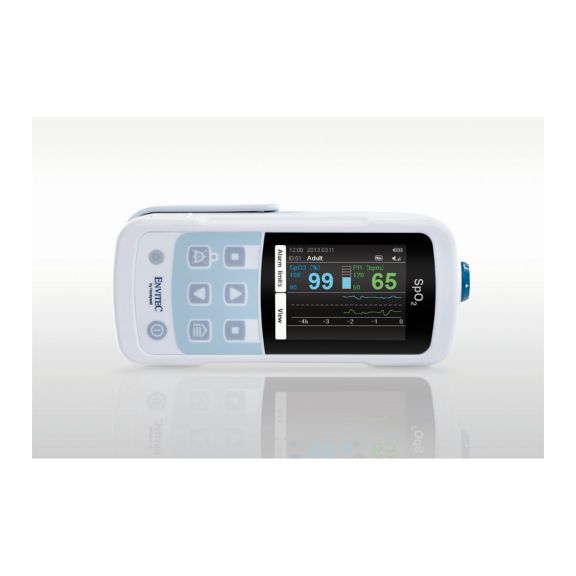
Ein Pulsoximeter – viele Optionen. Geeignet für die kontinuierliche und stichprobenartige SpO2-Überwachung. Als tragbares oder stationäres Gerät konzipiert. Misst SpO2, Pulsfrequenz und zeigt die plethysmographische Pulswelle an.
EnviteC-Querverweisliste | Technische Unterstützung
Die Pulsoximetrie ist eine nicht-invasive, einfache und zuverlässige Technologie zur Messung der funktionellen Sauerstoffsättigung des arteriellen Blutes (SpO2) und der Pulsfrequenz.
Die Verfügbarkeit von kleinen, tragbaren und handgeführten Pulsoximeter-Monitoren eröffnet zahlreiche und unterschiedliche Anwendungsbereiche im Gesundheitswesen. SpO2-Sensoren sind das Kernelement in der Praxis. Sie werden an den entsprechenden Körperstellen des Patienten angebracht.
MySign® S ist eine intelligente Lösung für die nicht-invasive Überwachung von Vitalparametern – ein einfach zu bedienender Monitor, der außergewöhnlich genaue Messwerte liefert.
EnviteC by Honeywell