Pulse Oximeter & Accessories
Pulse Oximeter & Accessories
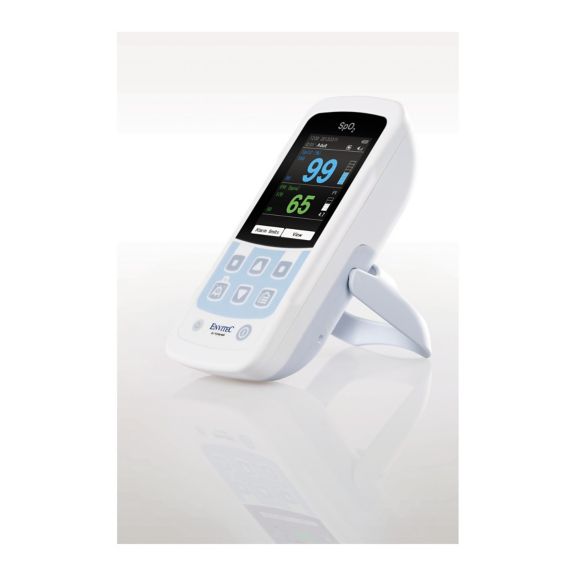
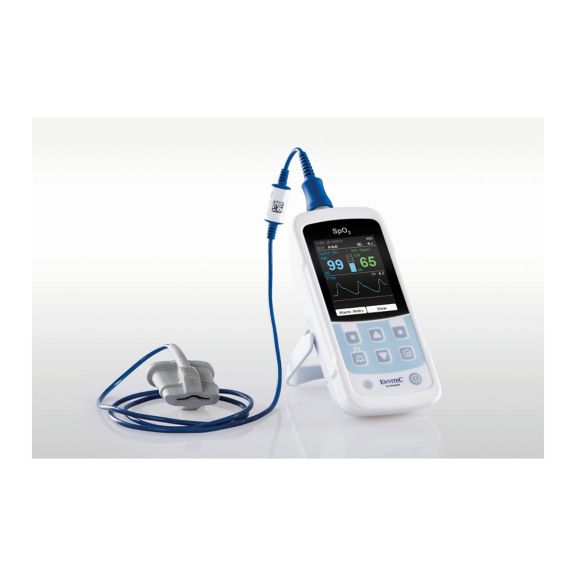
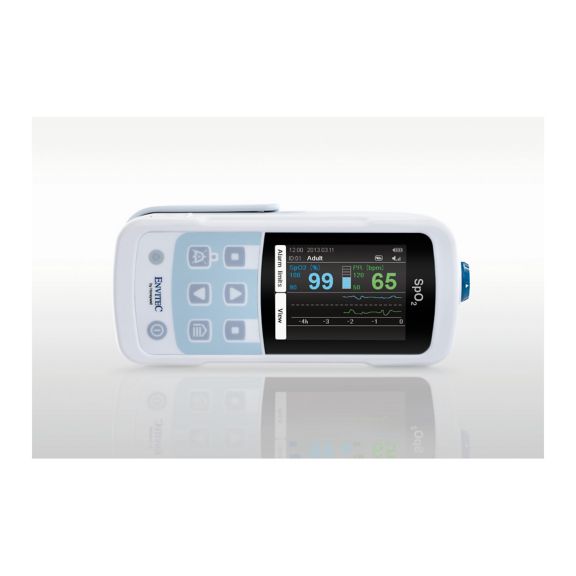
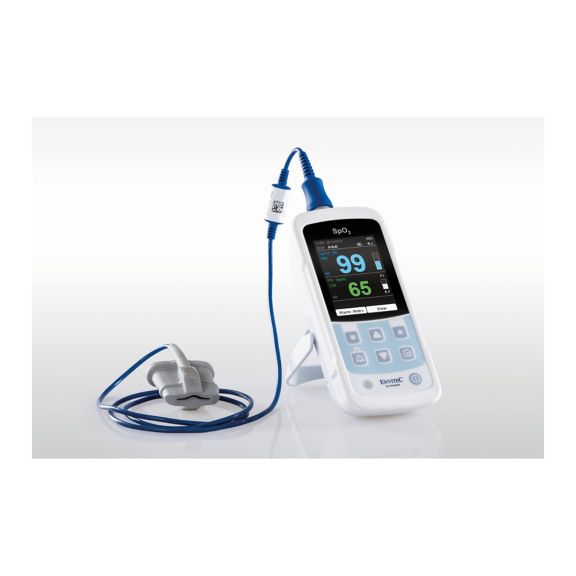
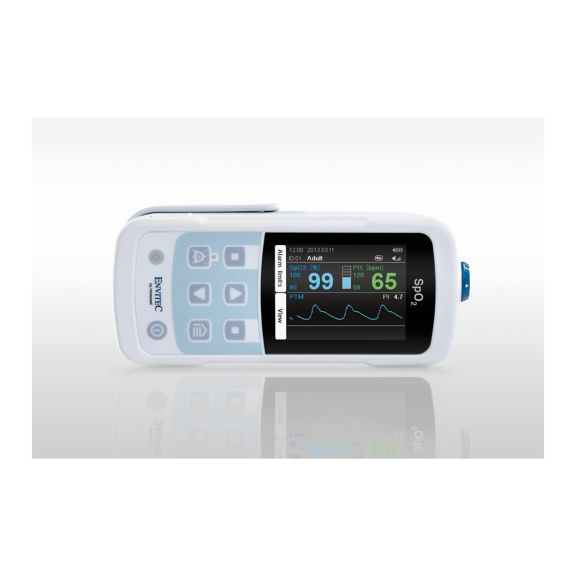
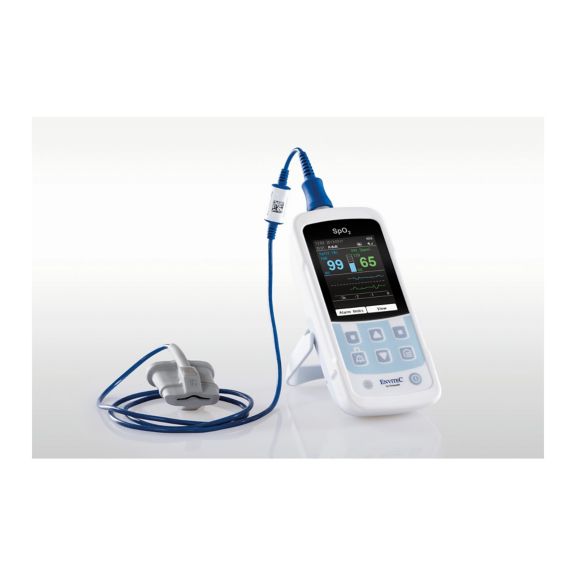
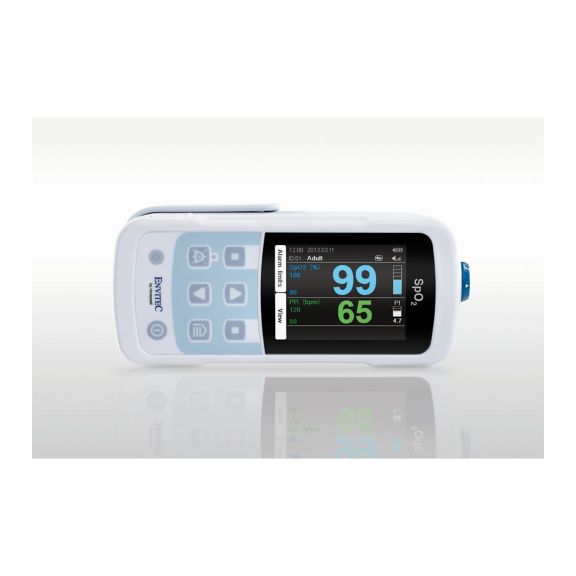
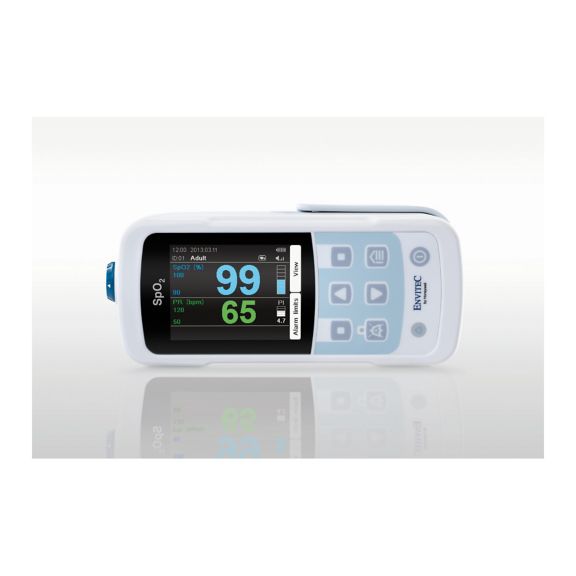
One pulse oximeter device – many options. Suited for continuous and spot check SpO2 monitoring. Designed as a portable or stationary device. Measures SpO2, pulse rate and displays the plethysmographic pulse wave
EnviteC Cross Reference List | Tech Support
Pulse oximetry is a non-invasive, simple and reliable technology that measures the functional oxygen saturation of arterial blood (SpO2) and the pulse frequency.
The availability of small, portable and handheld pulse oximeter monitors offers numerous and different application areas in healthcare environments. SpO2 sensors are the core element in practice. They are applied to the corresponding body parts of the patient.
MySign® S is an intelligent solution for non-invasive vital sign monitoring – an easy-to-use monitor that provides exceptionally accurate readings.
EnviteC by Honeywell