再使用可能なパルスオキシメータ用センサ
SoftTip®
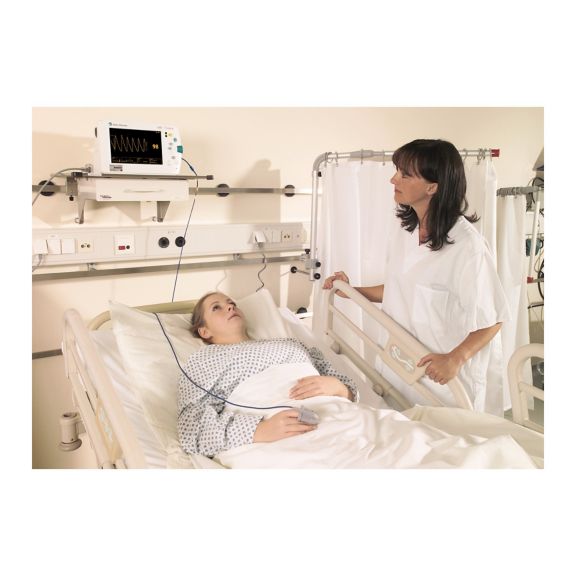
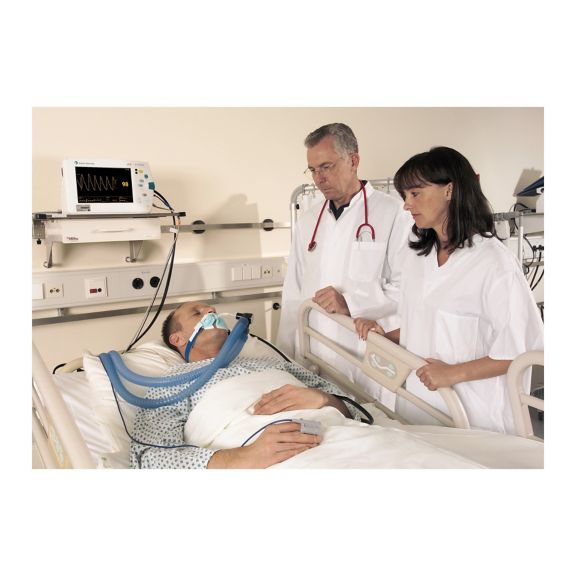
再利用可能なパルスオキシメータ用センサSoftTip®は、取り扱いが簡単で、洗浄・消毒に適した設計です。応力の影響を最小限に抑えます。あらゆる用途に対応できるサイズを取り揃え、快適な着け心地を実現しました。
洗浄液に浸すことで簡単に洗浄でき、長期間にわたって再利用可能なフィンガーセンサです。また、高い機械的ストレス耐性を備えています。
独自の製品設計により、患者さんの快適な装着感と正確な測定結果を実現しました。ほぼすべての主要メーカーの一般的なモニタリング機器との互換性があります。
ハネウェル製EnviteC