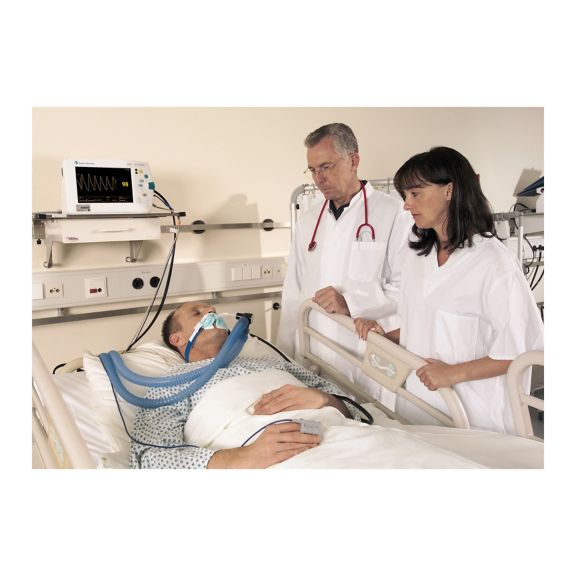
재사용 가능 맥박 산소측정장치 센서
SoftTip®
손쉬운 취급, 최적의 세척 및 철저한 소독을 통해 재사용 SoftTip® 맥박 산소측정 센서. 기계적 응력에 대한 최대 저항. 모든 응용 분야에 다양한 크기로 착용이 매우 편안함
세정액에 완전히 담가 세척이 용이한 장기 재사용 가능 센서입니다. 또한 최대 기계적 응력을 견뎌냅니다.
독특한 제품 설계로 매우 우수한 환자의 편안함과 매우 정확한 측정 결과를 보장합니다. 대부분의 유명 제조업체에서 제공하는 일반적인 모니터링 장치와의 호환성이 보장됩니다.
Honeywell의 EnviteC